Your cart is empty.
shop now
Your cart is empty.
shop now
Thermogravimetric Analysis (TGA) is an invaluable analytical technique used to measure changes in the weight of a sample as a function of temperature or time. Its applications span diverse fields, including material science, pharmaceuticals, and environmental analysis.
For accurate and reliable TGA measurements, it is crucial to understand the various factors that can influence the results. In this comprehensive article, we will delve into the key factors that affect the accuracy and precision of TGA measurements.
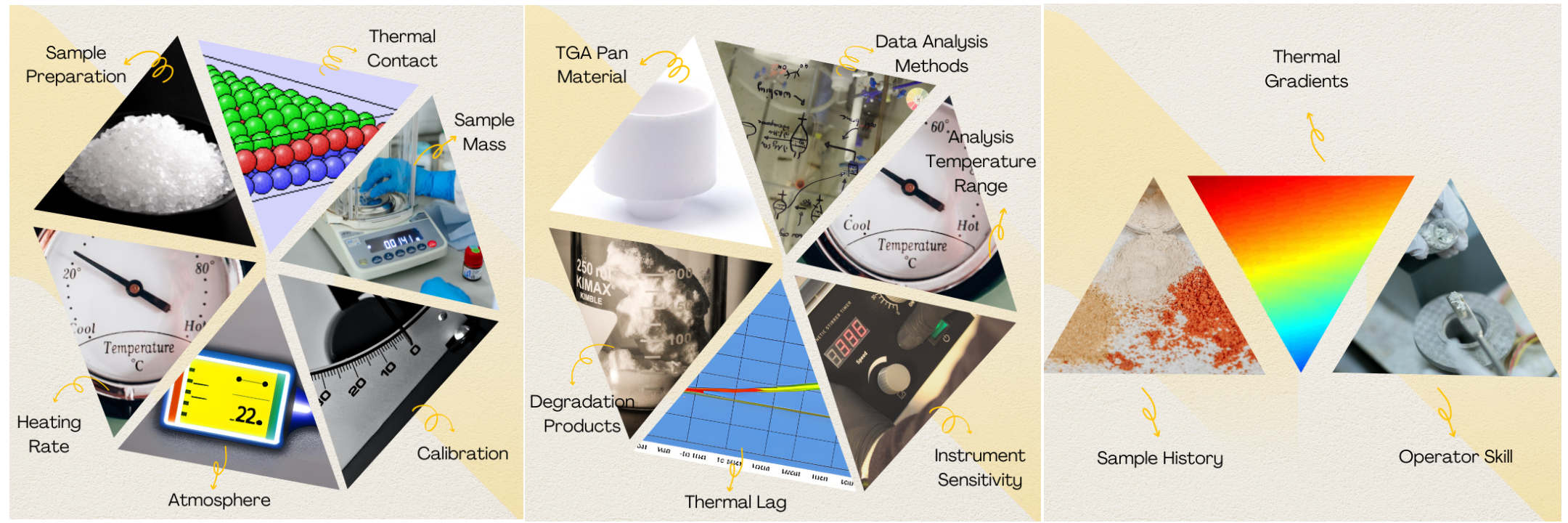
The factors that influence the accuracy and precision of TGA measurements
Sample preparation is the foundation of successful TGA analysis. It involves ensuring that the sample is representative, homogenous, and free from contaminants or impurities. Inhomogeneous samples can lead to inconsistent weight changes, skewing the results. To achieve accurate measurements, researchers should take meticulous care during sample collection, handling, and preparation.
The heating rate in TGA determines how fast the temperature increases during the analysis. Selecting the appropriate heating rate is crucial as it directly impacts the precision of weight change measurements. Slow heating rates allow for better equilibration of the sample, resulting in more accurate readings. However, slow rates might miss certain rapid transformations. On the other hand, high heating rates can cause thermal gradients within the sample, leading to inaccuracies. Researchers must carefully choose a heating rate that suits the specific nature of their samples and the phenomena they aim to study.
The surrounding atmosphere during TGA significantly influences the results. Different gases or ambient conditions can cause variations in the weight change of the sample. The choice of atmosphere depends on the material being analyzed and the specific objectives of the study. Inert gas, such as nitrogen, is commonly used to avoid unintended reactions, while oxidizing or reducing atmospheres can be employed to study specific chemical transformations.
Regular calibration of the TGA instrument is paramount to ensure accuracy and consistency in measurements. Calibration corrects any deviations in the baseline and ensures that the instrument provides precise weight change data. Periodic calibration using standard reference materials is essential to maintain the reliability of TGA instruments.
The quantity of sample used in TGA analysis can impact the sensitivity and accuracy of measurements. Insufficient sample mass might lead to weak signals, making it challenging to detect small weight changes accurately. Conversely, an excessive sample mass can overload the instrument, affecting its performance and introducing errors. Researchers should carefully optimize the sample mass to achieve optimal TGA results.
Proper thermal contact between the sample and the TGA pan is essential for accurate measurements. Inadequate contact can result in incomplete heat transfer, leading to discrepancies in weight change readings. To ensure good thermal contact, researchers should follow proper loading procedures and use appropriate pan materials.
The choice of TGA pan material can influence the measurements. Different pan materials possess varying thermal conductivities and might interact differently with the sample. Researchers should choose a pan material that minimizes any potential effects on the sample and provides consistent and reliable data.

During TGA analysis, samples might undergo degradation and produce by-products. These by-products can further interact with the sample, leading to changes in weight measurements. Researchers should be aware of potential degradation reactions and consider their impact on the accuracy of TGA results.
Thermal lag refers to the delay in the sample's temperature relative to the surrounding environment. This phenomenon can affect the accuracy of weight change measurements, particularly during rapid temperature changes. Minimizing thermal lag through proper heating rate selection and equipment calibration is essential to improve the precision of TGA analysis.
The sensitivity of the TGA instrument plays a crucial role in the accuracy of measurements. The instrument's sensitivity determines its ability to detect small weight changes reliably. Too much sensitivity can result in noise in the data, while insufficient sensitivity might miss important weight changes. Striking the right balance is essential for obtaining high-quality TGA data.
Selecting the appropriate temperature range for TGA analysis is vital. Some transformations or degradation events might occur at temperatures beyond the chosen range, leading to incomplete or inaccurate data. Researchers should carefully consider the nature of the sample and the phenomena of interest to set the correct temperature range for analysis.
The methods used to analyze TGA data can significantly influence the interpretation of results. Proper data analysis techniques ensure that meaningful conclusions can be drawn from the measurements. Misinterpretation of data due to inappropriate analysis methods can lead to erroneous conclusions.
The history of the sample, including storage conditions and any prior treatments, can impact TGA measurements. Changes in the sample due to its history must be considered to avoid misinterpretation of weight change data.
Thermal gradients within the TGA instrument can introduce errors in measurements. Uneven temperature distributions may affect the sample's behavior during the analysis, leading to inaccuracies in weight change readings.
The skill and experience of the operator conducting the TGA experiments significantly impact the reliability of measurements. A well-trained operator can ensure proper technique, minimize experimental errors, and obtain high-quality data.
Thermogravimetric Analysis (TGA) is a powerful analytical technique that provides valuable insights into weight changes in materials. To achieve accurate and precise TGA measurements, researchers must consider various factors, such as sample preparation, heating rate, atmosphere, calibration, sample mass, thermal contact, TGA pan material, degradation products, thermal lag, instrument sensitivity, analysis temperature range, data analysis methods, sample history, thermal gradients, and operator skill. By understanding and optimizing these factors, researchers can unlock the full potential of TGA analysis and further advance their understanding of materials and their thermal behavior.
Yes, TGA can be employed to analyze complex materials and study their thermal properties and behavior.
Common calibration standards include certified reference materials, such as pure metals or well-characterized polymers.
Yes, TGA can provide valuable data for studying the kinetics of decomposition reactions by analyzing the weight loss over time.
The initial weight of the sample is essential as it provides a baseline for calculating weight changes during the TGA experiment.
Yes, TGA data can provide insights into the thermal stability of materials, aiding in predicting their performance under specific conditions.