Your cart is empty.
shop now
Your cart is empty.
shop now
A Thermogravimetric Analysis (TGA) crucible is a pivotal apparatus within the realm of thermal analysis, specifically designed to elucidate the intricate aspects of a material's thermal stability and degradation kinetics. Its role extends beyond mere containment, acting as a controlled vessel through which samples undergo carefully orchestrated temperature variations to unveil their thermal behavior in an evolving environment.
Here's an in-depth exploration of how a TGA crucible functions in determining degradation kinetics:
The meticulous process commences with the precise allocation of the substance under investigation into the TGA crucible. This material might encompass polymers, complex chemical compounds, or any substance necessitating an in-depth thermal assessment. The crucible's selection hinges upon its capacity to withstand formidable temperatures and its chemical inertness, effectively preventing any unintended reactions with the sample.
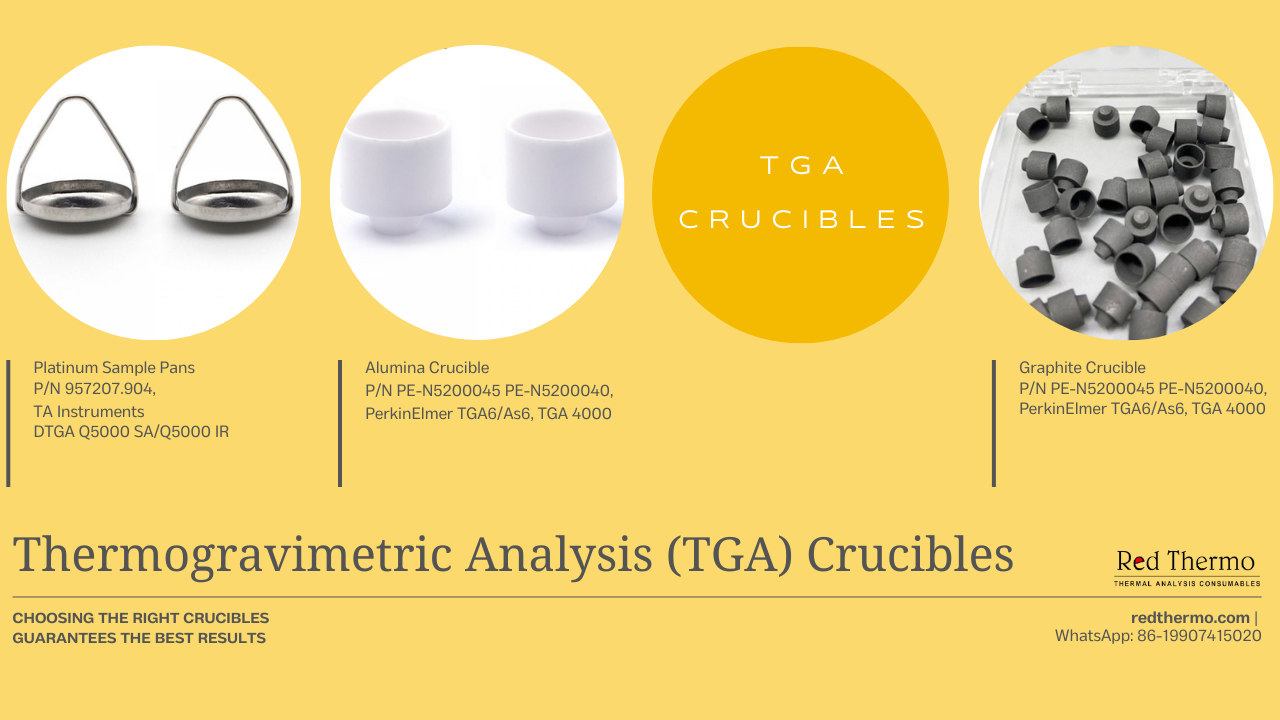
TGA crucibles are ingeniously constructed from materials boasting resilience in the face of high thermal stresses and an innate resistance to chemical interactions. Common choices encompass alumina, graphite, and platinum, each contributing to a controlled environment where sample interactions are curtailed.
At the heart of the experiment lies the TGA instrument, housing the crucible, along with a highly sensitive balance. This balance orchestrates the accurate quantification of the initial mass of the sample-laden crucible, establishing a reference point for the forthcoming analysis.
A carefully devised heating program is instigated within the TGA instrument, orchestrating a gradual and precisely controlled ascent in temperature. As the temperature gradient intensifies, the sample within the crucible navigates a labyrinth of chemical transformations, prominently including degradation phenomena.
As thermal decomposition or degradation unfolds, volatile components might be liberated, engendering shifts in the sample's mass. The TGA apparatus, equipped with sensitive sensors, diligently records even the most minuscule weight fluctuations. This meticulous data acquisition is a cornerstone for a nuanced analysis.
The recorded dataset metamorphoses into a canvas upon which researchers paint the intricate portrait of the material's degradation kinetics. The rate of mass alteration, intricately entwined with temperature variations, unveils multifaceted degradation stages: the initial phase of volatilization, subsequent decomposition events, and potential oxidation occurrences. By identifying these phases, scientists gain invaluable insights into the material's stability and propensity for thermal degradation.
To transcend the analysis, researchers employ varying heating rates. This strategic variation provides the data needed for applying the Arrhenius equation, a mathematical construct pivotal in deducing activation energies and pre-exponential factors governing the degradation reactions. These quantitative values effectively unveil the energy barriers dictating reaction rates, thus casting a more profound light upon the material's degradation kinetics.
In essence, a TGA crucible transforms into a microcosm of controlled experimentation, accommodating samples in their transformative journey through varying temperatures. The resulting weight measurements crystallize as a testament to the material's thermal behavior, its degradation kinetics, and the underlying chemical processes. Such insight-rich investigations serve as a cornerstone in diverse domains including materials science, polymer engineering, and pharmaceutical advancements, where understanding and optimizing thermal stability is paramount.