Your cart is empty.
shop now
Your cart is empty.
shop now
The sample handling and netzsch sample crucible selection for Netzsch STA 449 Jupiter will be covered in this article
When preparing the sample for interpretation, it is essential to ensure its consistency and good thermal contact between the sample and heat-flux sensor for the best results.
The techniques most commonly employed for the preparation of solid and liquid samples are outlined below.
Powered Solids | The sample is spread evenly at the bottom of the sample crucible. |
Compact solids |
Thin slices of materials such as rubber or thermoplastic can be cut with a knife, scalpel, or razor blade.
A hollow drill is employed to remove a sample disc of an appropriate size from the larger disc at its center for the analysis. If a disc of the required size cannot be punched out, thin slices of the material should be placed on the bottom of the crucible.
It is essential to utilize a sample crucible, since applying the sample material directly can lead to contamination of the sensor. |
Films |
A hollow drill or a pair of punch pliers can be used to cut discs out of films. The discs should be large enough to completely cover the base of the crucible.
For better contact between the sample and the bottom of the crucible, the lid should be placed concave side down and sealed. |
Fibers |
The fiber is cut into small pieces and spread in a parallel pattern on the bottom of the crucible.
The small rod is wound with the fiber, and then the coiled fiber is taken off the rod and put into the crucible.
A crucible is filled with a voluminous fiber material that is wrapped in aluminum foil and cut at both ends. The weight of the sample can be increased with additional fiber material.
The addition of a drop of silicone oil to all cases can improve the heat transfer and thus enhance the significance of the experimental results. |
Liquids |
Samples of liquid with varying viscosity can be placed in a crucible using a thin glass rod, a micro-pipette, or a syringe. |
Unstable samples |
Special pressure-tight crucibles (optional) are used to test unstable samples. If these are employed, it is necessary to recalibrate the measuring unit. |
Evaporation reaction |
When investigating evaporation reactions, e.g. evaporation of water of crystallization, a closed crucible with a small hole in the center of the lid should be used. Before sealing the hole, it should be punched to stop the crucible from becoming misshapen. |
The table below provides the most up-to-date information; however, it is not comprehensive. Please use it as a reference only.
For platinum crucibles with Al2O3 in liners, the same data applies as that for corundum crucibles, except for nickel as a calibration substance. As the melting point of nickel is 1455°C, which is above the maximum recommended operating temperature for Pt crucibles on a type S sample carrier, it should not be used.
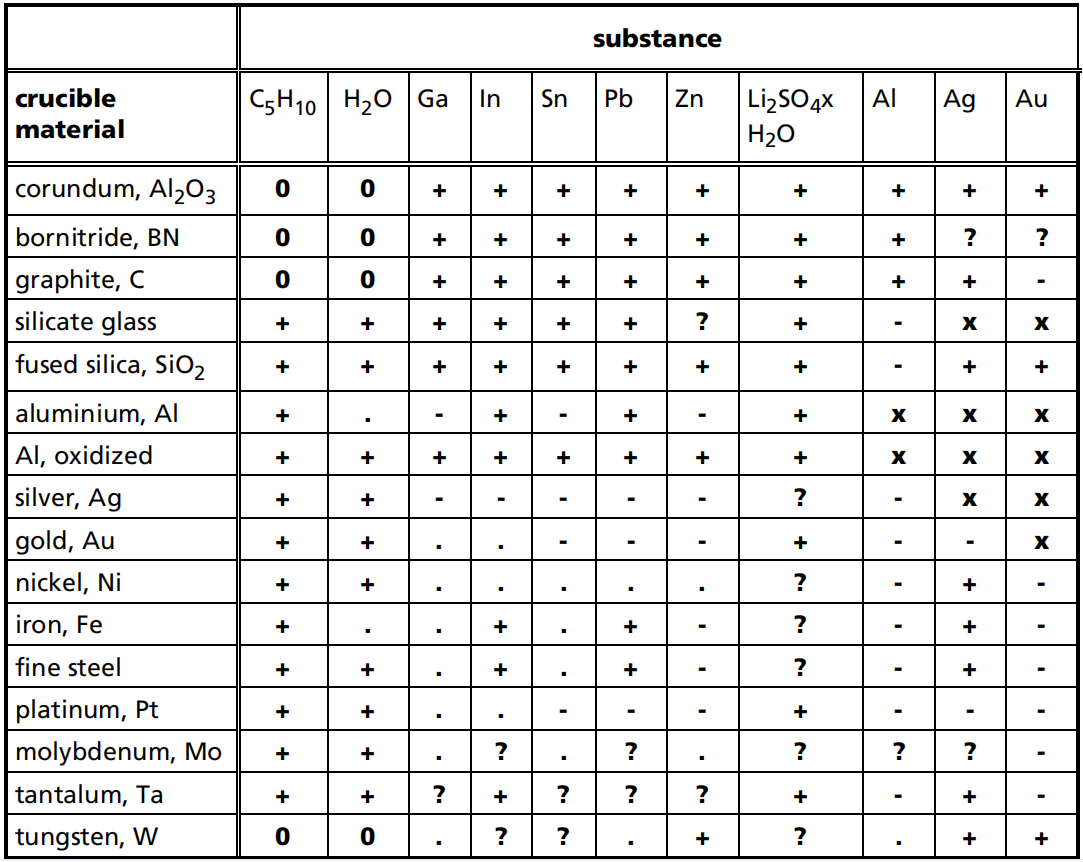
Source: Die Temperaturkalibrierung dynamischer Kalorimeter II. Kalibriersubstanzen, H. K. Cammenga, PTB-Mitteilungen 102 1/92
+ no solubility, no influence on the melting temperature
- melt dissolves the crucible material, significant change of melting temperature
? partial solution process, change of melting temperature may be ignored
x crucible melts
? compatibility unknown
0 combination cannot be realized
P/N. and descriptions of Sample crucibles for STA 449 Jupiter are listed below.
Sample Crucible for STA 449 Jupiter *supplied by RedThermo | |
Part No. | Description |
85μl alumina crucible | |
Alumina crucible for sample carrier 3.4ml | |
0.2ml alumina crucible | |
0.3ml alumina crucible | |
0.15ml alumina crucible | |
0.3ml graphite crucible | |
85μl graphite crucible | |
30/40 μl Aluminum concavus pan | |
85μl Platinum sample pan | |
25/40μl aluminum crucible/lid(cold weldable) | |