Your cart is empty.
shop now
Your cart is empty.
shop now
The principle of thermogravimetric analysis is based on the fact that the mass of a material is affected by changes in temperature. The resulting weight changes can be used to gain insight into the thermal decomposition of a material, or other changes in its properties. This article will discuss the principle of thermogravimetric analysis and the various factors that can affect the results.
Thermogravimetric (TG) is a technique that measures the mass of a sample under program temperature control as it changes with temperature or time.
Thermogravimetric analysis mainly studies the thermal stability, thermal decomposition reaction, and oxidation degradation of materials in inert gases, air, and oxygen; it is also widely used to study all physical processes involving mass change, such as determining the moisture, volatiles and residues, adsorption and desorption, vaporization rate and heat of vaporization, sublimation rate and heat of sublimation, composition of polymers or blends with fillers, etc.
Plotting a thermogravimetric curve (TG curve) of weight fraction w against temperature T or time t: w = f (T or t), since it is mostly linear heating, the difference between T and t is only a constant. The first order derivative of the TG curve with respect to temperature or time, dw/dT or dw/dt, is called differential thermogravimetric curve (DTG curve). (Figure 1.)
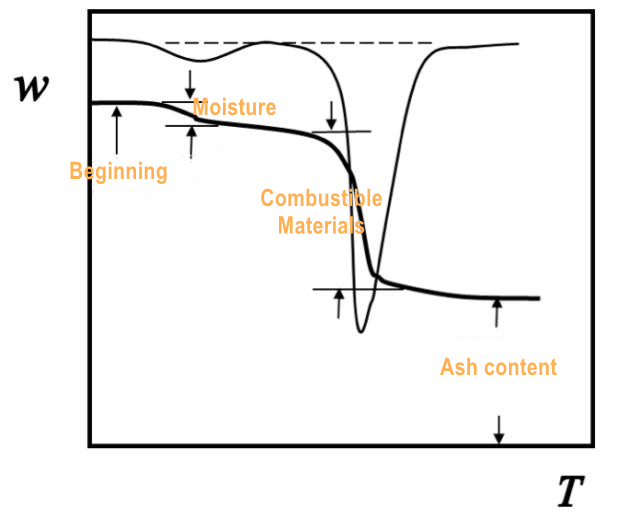
(Figure 1.)
At point B in the figure below, the cumulative weight change reaches the lower limit of the thermobalance detection, which is called the reaction initiation temperature; at point C, Tf, the change of weight can no longer be detected, which is called the reaction termination temperature; Ti or Tf can also be determined by extrapolation and divided into points G and H; or the temperature when the weight loss reaches a predetermined value (5%, 10%, etc.) can be taken as Ti. Tp represents the maximum weight loss rate temperature, which corresponds to the peak temperature of the DTG curve. The area of the peak is proportional to the weight change of the sample. (Figure 2.)
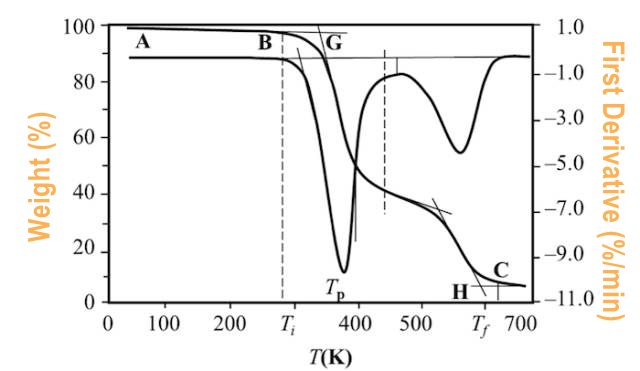
(Figure 2.)
The faster the heating rate, the greater the temperature lag, the higher the Ti and Tf, and the wider the reaction temperature range. It is suggested that the polymer sample is 5~10K/min, and the inorganic and metallic sample is 10~20K/min. (Figure 3.)
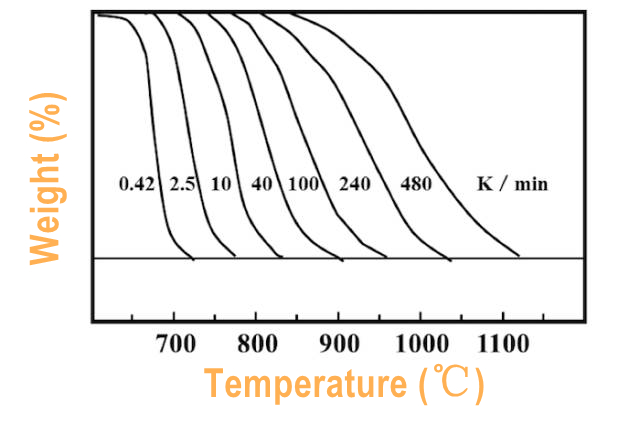
(Figure 3.)
The particle size of the sample should not be too large, and the degree of packing should be moderate. For the same batch of test samples, the particle size and packing tightness should be consistent for each sample.
(Figure 4.)
Common atmospheres include air and N2, and also O2, He, H2, CO2, Cl2 and water vapor. Different atmospheres have different reaction mechanisms. When the atmosphere reacts with the sample, the shape of the TG curve is affected.
For example, when PP is used with N2, oxidation gains occur in an atmosphere-free environment at 150 to 180°C. It is necessary to consider if there is any chemical reaction between the atmosphere and the thermocouple, sample container or instrument components, and if there is any risk of explosion and poisoning.
When the atmosphere is dynamic, attention should be paid to the influence of flow rate on temperature measurement accuracy, with the flow rate of 40-50 mL/min. If there is re-condensation of volatile substances, the ventilation of the balance chamber should be increased.
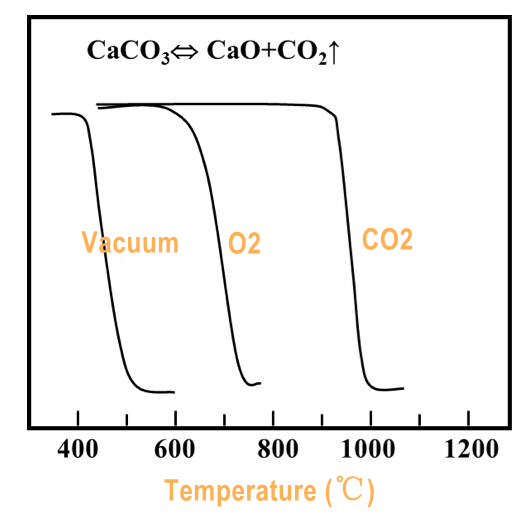
(Figure 5.)
(1) The material of the sample pan includes glass, aluminum, ceramics, quartz, metal, etc.
(2) The sample pan should be inert to the sample, intermediates, and final products.
(3) Samples of polytetrafluoroethylene (PTFE) cannot be placed in ceramic, glass or quartz sample pans, as they will form volatile carbides with each other.
(4) Platinum sample pans are not suitable for samples of polymers containing phosphorus, sulfur or halogens, because platinum has hydrogenation or dehydrogenation activity for such substances.
(5) When selecting the sample dish, it is better to use a shallow plate. When testing, spread the sample thinly on the bottom of the dish to facilitate heat transfer and the diffusion of products.

Different Curie temperature magnetic materials can be used for thermobalance. At Curie Point, there is an apparent loss of weight. (Figure 6.)
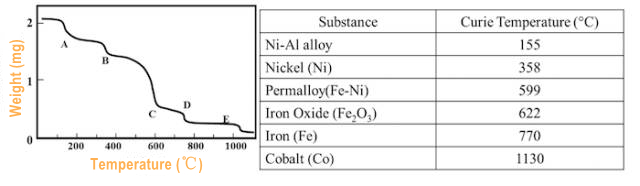
(Figure 6.)